Kasper Hansen
Associate Professor, Neuroscience
Contact Information
- Department:
- Division Of Biological Sciences
- Email:
- kasper.hansen@umontana.edu
- Phone:
- (406) 243-4820
- Personal Website:
- https://hansen-neurolab.com/
Office Address
ISB 216
32 Campus Dr MS 1552
Missoula MT, 59812
After completing a B.Sc. in Chemistry and a M.Sc. in Molecular Biology at University of Aarhus in Denmark, Kasper Hansen received his Ph.D. in Molecular Pharmacology from University of Copenhagen, Denmark in 2006. Following a postdoctoral fellowship in the Department of Pharmacology, Emory University School of Medicine, he moved to the University of Montana in 2013. Kasper Hansen is a faculty in the Division of Biological Sciences, the Center for Biomolecular Structure and Dynamics (CBSD), and the Center for Structural and Functional Neuroscience (CSFN).
More information on the research program and publications can also be found here: Kasper Hansen lab.
Research Interests
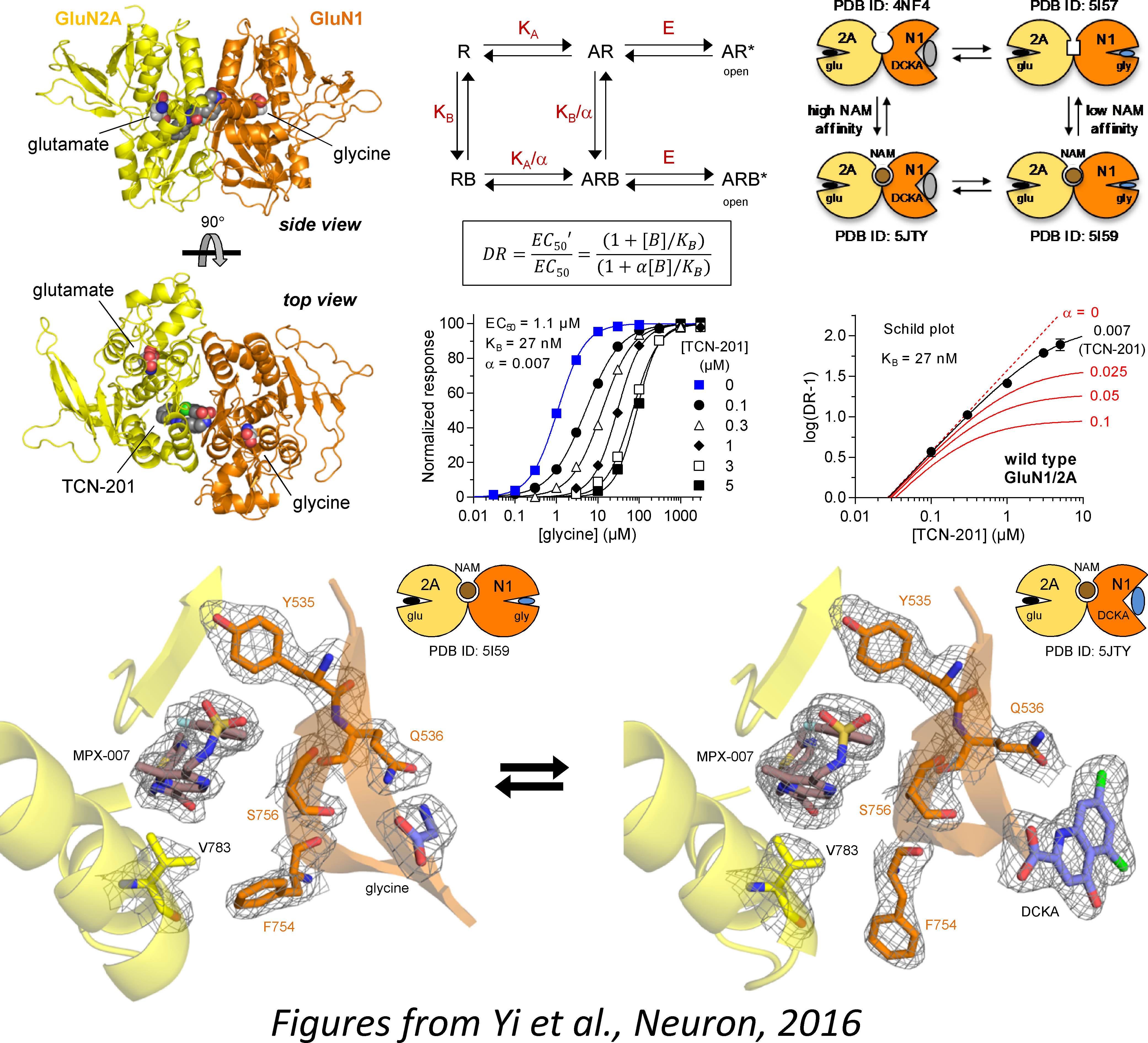
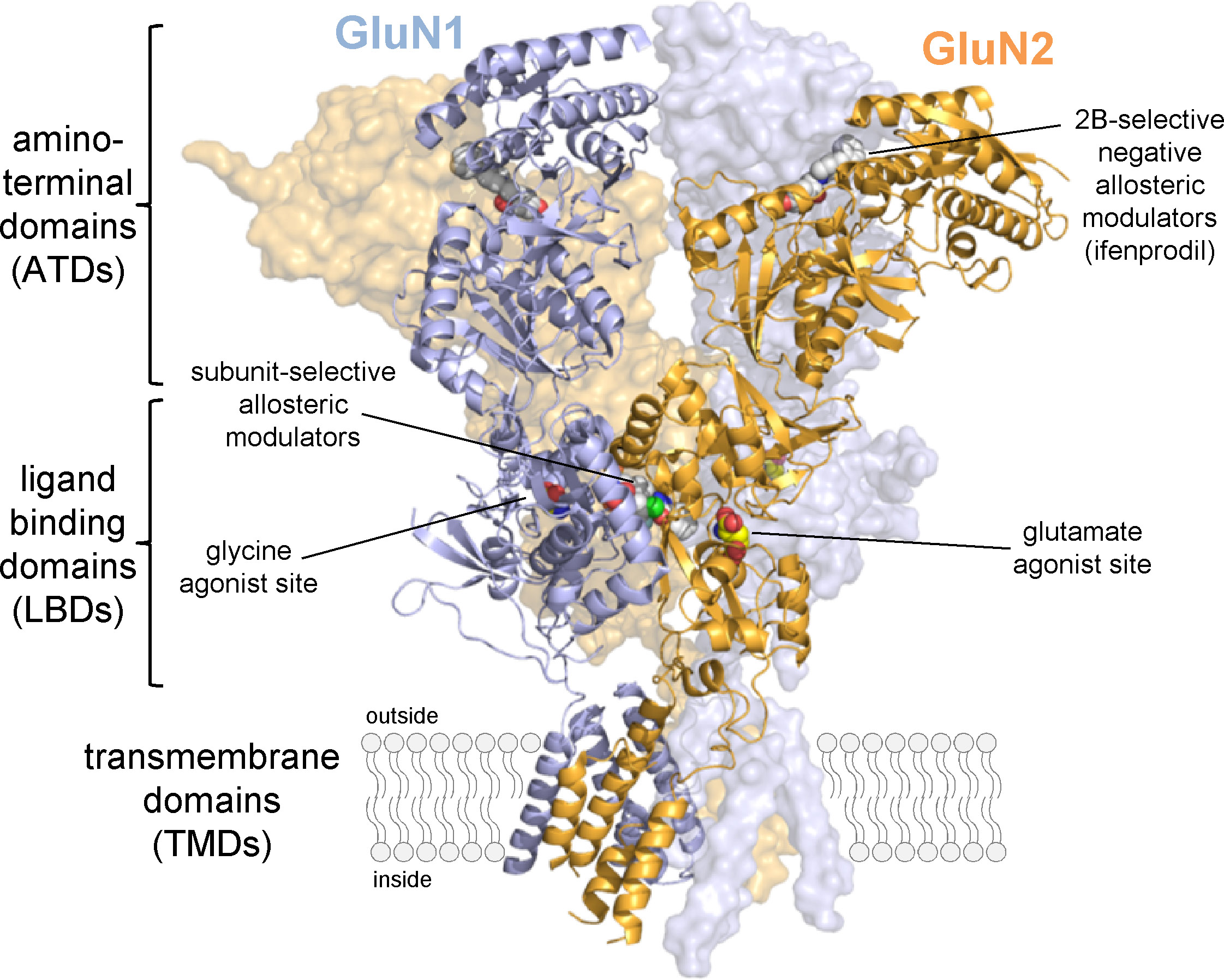 When glutamate is released from the presynaptic membrane during fast excitatory neurotransmission, it readily diffuses across the synaptic cleft and activates ligand-gated ion channels present at the postsynaptic membrane. These ionotropic glutamate receptors have been divided into three classes based on agonist pharmacology and structure, namely AMPA, kainate, and NMDA receptors. NMDA receptors are critically involved in many important neuronal functions, including frequency encoding of information, synaptic plasticity, and neuronal development. However, NMDA receptors also play overt roles in a variety of neurological and psychiatric disorders in the central nervous system, including depression, schizophrenia, ischemia, seizures, traumatic brain injury, Parkinson’s, Huntington’s, and Alzheimer’s diseases. For these reasons, there has been widespread interest in understanding structure, function, and regulation of NMDA receptors for the purpose of developing new treatments for a number of diseases. The overarching goal of research in the laboratory is to enhance the synthetic pharmacology of NMDA receptors (i.e. to develop novel agonists, antagonists, and modulators), and to identify and validate new strategies and targets that can be exploited for therapeutic intervention. The laboratory uses a multidisciplinary approach (electrophysiology, synthetic biology, molecular screening, biochemistry, structural biology, and molecular modeling) to advance our understanding of the relationships between structure, function, and pharmacology of NMDA receptors. These efforts are mainly been divided into four areas:
When glutamate is released from the presynaptic membrane during fast excitatory neurotransmission, it readily diffuses across the synaptic cleft and activates ligand-gated ion channels present at the postsynaptic membrane. These ionotropic glutamate receptors have been divided into three classes based on agonist pharmacology and structure, namely AMPA, kainate, and NMDA receptors. NMDA receptors are critically involved in many important neuronal functions, including frequency encoding of information, synaptic plasticity, and neuronal development. However, NMDA receptors also play overt roles in a variety of neurological and psychiatric disorders in the central nervous system, including depression, schizophrenia, ischemia, seizures, traumatic brain injury, Parkinson’s, Huntington’s, and Alzheimer’s diseases. For these reasons, there has been widespread interest in understanding structure, function, and regulation of NMDA receptors for the purpose of developing new treatments for a number of diseases. The overarching goal of research in the laboratory is to enhance the synthetic pharmacology of NMDA receptors (i.e. to develop novel agonists, antagonists, and modulators), and to identify and validate new strategies and targets that can be exploited for therapeutic intervention. The laboratory uses a multidisciplinary approach (electrophysiology, synthetic biology, molecular screening, biochemistry, structural biology, and molecular modeling) to advance our understanding of the relationships between structure, function, and pharmacology of NMDA receptors. These efforts are mainly been divided into four areas:
Pharmacology and function of triheteromeric NMDA receptors
There are seven NMDA receptor subunits (GluN1, GluN2A-D, and GluN3A-B) that assemble as tetrameric receptors. Recombinant studies almost exclusively describe diheteromeric receptors assembled from GluN1 and one type of GluN2, but at least three different subunits have been identified in most, if not all, NMDA receptor-expressing cells, and the majority of native receptors are triheteromers in that they contain three different subunits. These triheteromeric NMDA receptors are poorly understood due to the problem that co-expression of three different subunits in heterologous expression systems will yield multiple different receptor populations. We recently developed a method to tightly control the subunit composition of NMDA receptors, thereby enabling selective expression of triheteromeric NMDA receptors. This new approach provides opportunities to develop therapeutic agents that target the disease-relevant native triheteromeric NMDA receptors and to study properties relevant to synaptic signaling.

For examples of studies on triheteromeric NMDA receptors, see:
- Triheteromeric GluN1/GluN2A/GluN2C NMDARs with Unique Single-Channel Properties Are the Dominant Receptor Population in Cerebellar Granule Cells.
- Properties of Triheteromeric N-Methyl-d-Aspartate Receptors Containing Two Distinct GluN1 Isoforms.
- Allosteric Interactions between NMDA Receptor Subunits Shape the Developmental Shift in Channel Properties.
- Distinct functional and pharmacological properties of triheteromeric GluN1/GluN2A/GluN2B NMDA receptors.
- Functional analysis of a de novo GRIN2A missense mutation associated with early-onset epileptic encephalopathy.
Structure and function of GluN3-containing NMDA receptors
Many basic questions related to the structure, function, and pharmacology of GluN3-containing NMDA receptors are unanswered. Despite the lack of basic understanding, we know from studies using GluN3-deficient and GluN3-overexpressing mice that GluN3 subunits are involved in synapse maturation, synaptic plasticity, and neuroprotection. They could therefore be promising new therapeutic targets in several neurological diseases that include excitotoxicity or cognitive impairment.

For examples see:
- Structure-based discovery of antagonists for GluN3-containing N-methyl-D-aspartate receptors.
- Negative allosteric modulation of GluN1/GluN3 NMDA receptors
Development of novel subunit-selective NMDA receptor ligands
For examples see:
- Structural basis of subunit selectivity for competitive NMDA receptor antagonists with preference for GluN2A over GluN2B subunits.
- Identification of AICP as a GluN2C-Selective N-Methyl-d-Aspartate Receptor Superagonist at the GluN1 Glycine Site.
- A subunit-selective potentiator of NR2C- and NR2D-containing NMDA receptors.
Mechanism and structural determinants of NMDA receptor modulators
For examples see:
- Structural Basis for Negative Allosteric Modulation of GluN2A-Containing NMDA Receptors.
- Design, synthesis, and structure-activity relationship of a novel series of GluN2C-selective potentiators.
- Subunit-selective allosteric inhibition of glycine binding to NMDA receptors.
- Structural and mechanistic determinants of a novel site for non-competitive inhibition of GluN2D-containing NMDA receptors.
Publications
See full publication list in PubMed and citation records in ResearchGate or Google Scholar.
More information on the research program and publications can be found here: Kasper Hansen lab.
Affiliations
Departments and Centers
- Division of Biological Sciences
- Center for Biomolecular Structure and Dynamics
- Center for Structural and Functional Neuroscience
Graduate Programs
These programs are part of the Molecular and Biomedical Sciences (MBS) umbrella.